The American Wigeon is a dabbling duck about the size of a Mallard, with a bluish bill.
Once threatened by widespread and protracted drought, the American Wigeon population has since increased due to habitat conservation and improved rainfall in its prairie breeding areas. While the mean lifespan for an American Wigeon is only about two years, individuals as old as 21 years have been recorded from banding studies.
American Wigeons are excellent swimmers, and can take flight from water almost instantly without a running start. Males are often aggressive during pair formation in late winter and spring, and during the breeding season, pairs will occupy either a whole pond or a portion of a larger pond.
Breeding Male
Males have pinkish-brown flanks, upperparts, and breast, a wide green patch behind the eye, and a cream-colored forehead. Length 20 in. Wingspan: 32 in.
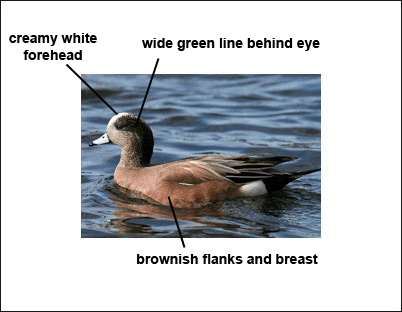

Males have pinkish sides, a pointed, black tail and blue bill with dark tip. The head is marked with a broad green area behind the eye and a white forehead. The white forehead gives it a widely used common name of “Baldpate.” Photograph © Alan Wilson.

This photo shows the dark mark behind the eye, variable in size and shape. Photograph © Elaine Wilson.

Male American Wigeon. Note the very fine markings on the feathers on the back. Photograph © Alan Wilson.

Preening male. Preening helps keep the feathers water-tight. Photograph © Elaine Wilson.

Standing male wigeon. The black tail and white of the upper wing coverts are clearly visible. Photograph © Alan Wilson.
On this page
Female
Females are also largely pinkish-brown, with a brownish-gray head and a dark area around the eye.

Females have pinkish to rusty sides, a dark tipped bill and dark area around the eye. Photograph © Alan Wilson.

The green speculum is just visible on this female. Rusty sides clearly visible. Photograph © Alan Wilson.

Female American Wigeon. Note the contrast in color between the head/neck and the breast. Photograph © Elaine Wilson.
Seasonal change in appearance
Males in nonbreeding plumage resemble females, but the green eye patch and cream forehead are still visible, though not as obvious.

Juvenile
The immature American Wigeon is similar to the adult female.
Habitat
American Wigeons inhabit ponds, lakes, and marshes, as well as salt bays.
Diet
American Wigeons primarily eat aquatic plants, but also some insects and waste grain.
Behavior
American Wigeons forage by gleaning food from the surface of the water or by submerging their head. They also graze on land.
Range
American Wigeons occur throughout much of the U.S. and Canada, breeding from the northern contiguous U.S. north to Alaska, and wintering across a broad swath of the central and southern U.S., as well as the Pacific states and provinces and the Atlantic Coast. The population has increased in recent decades.
Photos

Two pair. The green on the head of the male can appear black, depending on the light and angle of view. Photograph © Steve Wolfe.

The broad green mark behind the eye can appear brown, as on this photo, or black, depending on the lighting. Photograph © Alan Wilson.

Ducks will often exercise their wings before flight. Photograph © Alan Wilson.

Under wing linings are pale. Photograph © Alan Wilson.

The white upper wing coverts are easily visible when the wigeon is in flight. The wing patch is grayer on females and immature wigeons. The speculum is green but not as bright as on the Green-winged Teal. Photograph © Glenn Bartley.

Note the colors and shape of the tail. Photograph © Alan Wilson.

Female, left. Male, right. Write your own caption.
Fun Facts
As a dabbling duck, American Wigeons are limited to surface feeding, but they sometimes steal food from diving ducks and coots.
Young wigeons eat significant numbers of insects, but soon shift to a largely plant-based diet.
Vocalizations
Female American Wigeons give a growling “quack” similar to Canvasbacks or Redheads. Males give a two or three syllable whistle.
Similar Species
Gadwall

Gadwall
The female Gadwall has a light head, contrasting with a darker, browner body, very similar to the female American Wigeon. White wing patch on Gadwall is not always seen when the duck is swimming (as on this image) but obvious in flight. The bill of the Gadwall is larger and in good light shows orange around the edges.
Northern Pintail

Northern Pintail
Female Northern Pintails are also plainer in appearance than the males, with the head and neck lighter than the back. The bill of the pintail is longer than the wigeon’s. Forehead more sloped than on wigeon.
Green-winged Teal

Photograph © Glenn Bartley.
The Green-winged Teal also has a green band extending back from the eye. It is a smaller duck, with reddish head and vertical white mark just below the head. Also compare the female of the two species.
The pinkish flanks help separate the American Wigeon from other dabbling ducks.
Nesting
The American Wigeon nest is a shallow depression lined with grasses, leaves, and down, and is situated on land or on an island.
Number: Usually lay 8-1.
Color: Olive
Incubation and fledging
The young hatch at about 23-24 days and leave the nest almost immediately, but cannot fly for 6-8 weeks.

