The brilliantly colored male Blackburnian Warbler adds a splash of color to the coniferous forests in which it nests. Blackburnian Warblers forage high in trees for insects and larvae, and migrate to South America for the winter.
While Blackburnian Warblers defend a territory during the breeding season, once the young are old enough to be on their own, both adults and young may join mixed species flocks that include Black-capped Chickadees.
On this page
Description of the Blackburnian Warbler
BREEDING MALE
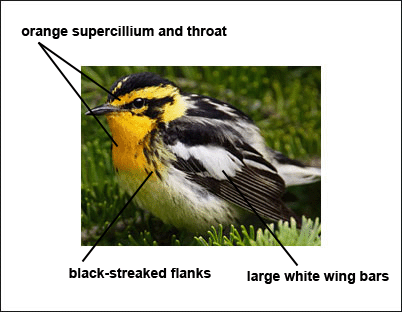
The Blackburnian Warbler has gray to black upperparts with long, whitish streaks, dark wings with two broad, white wing bars, a dark line through the eye, and a dark patch behind the eye.
Males have a black crown, bright orange supercilium that curves down around the side of the neck, a bright orange throat, streaked flanks, and very wide wing bars that meet, forming a large white patch. Length: 5 in. Wingspan: 8 in.
Female
Females are similar in pattern to the males, but are yellowish-orange rather than bright orange, have two distinct wing bars, and a grayer crown.
Seasonal change in appearance
Fall and winter birds are similar in pattern, but considerably duller in color.
Juvenile
Immatures are similar to fall adults, but duller.
Habitat
Blackburnian Warblers inhabit boreal forests, but during migration can occur in a variety of woodlands.
Diet
Blackburnian Warblers eat insects.

Photograph © Glenn Bartley.
Behavior
Blackburnian Warblers forage among small, treetop branches.
Range
Blackburnian Warblers breed in southeastern Canada, the northeastern U.S., and the Appalachians. They winter mostly in South America. The population appears stable.
More information:
Bent Life History
Visit the Bent Life History for extensive additional information on the Blackburnian Warbler.
Fun Facts
Nesting Blackburnian Warblers are very difficult to study, in part because their nests can be 80 feet up in a tree.
Blackburnian Warblers readily exploit occasional outbreaks of the spruce budworm.
Vocalizations
The song is a series of extremely high notes. A sharp flight call is also given.
Similar Species
Female Cerulean Warblers have a unique bluish color to the upperparts. The whitish streaks on the back of Blackburnian Warblers are distinctive.
Northern Parula
The Northern Parula does have an orangish breast, but has solid face pattern with blue-gray head.
Nesting
The Blackburnian Warbler’s nest is a cup of bark fibers, twigs, and grasses, and is lined with moss, lichens, and other materials. It is placed high on an outer branch of a conifer.
Number: Usually 4.
Color: Whitish with darker markings.
Incubation and fledging:
The young hatch at about 12-13 days and fledge at an unknown age, perhaps about 12 days, though remaining dependent on the adults for some time.
Bent Life History of the Blackburnian Warbler
Published by the Smithsonian Institution between the 1920s and the 1950s, the Bent life history series of monographs provide an often colorful description of the birds of North America. Arthur Cleveland Bent was the lead author for the series. The Bent series is a great resource and often includes quotes from early American Ornithologists, including Audubon, Townsend, Wilson, Sutton and many others.
Bent Life History for the Blackburnian Warbler – the common name and sub-species reflect the nomenclature in use at the time the description was written.
DENDROICA FUSCA (Muller)
Bagg and Eliot (1937) give the following account of the history of the naming of the Blackburnian Warbler:
Some time in the later eighteenth century, a specimen (apparently female) was sent from New York to England, and there described and named for a Mrs. Blackburn who collected stuffed birds and was a patron to ornithology. Blackburaiae: Gmelin’s latinization, In 1788, of this English name: was its scientific designation until quite recently, when in an obscure German publication, dated 1776, were discovered a description of a specimen from French Guiana (which Is well east of the species’ normal winter range), and the name fusca, blackish. Wilson recognized the male as a rare transient near PhIladelphia, but when he shot a female (apparently, though he called it a male) in the Great Pine Swamp, Pa., he named it Sylvia pai-u8, the Hemlock Warbler. Audubon, too, considered the Blackburnian and Hemlock WarNers distinct.”
Blackburnian seems to be a doubly appropriate name, for its upper parts are largely black and its throat burns like a brilliant orange flame amid the dark foliage of the hemlocks and spruces. A glimpse of such a brilliant gem, flashing out from its sombre surroundings, is fairly startling.
Throughout most of the eastern half of the United States the Blackburnian warbler is known only as a migrant, mainly from the Mississippi Valley eastward. Its summer range extends from Manitoba eastward to Nova Scotia, from Minnesota to New England, and southward in the Allegheny Mountains to South Carolina and Georgia, in the Lower Canadian and Upper Transition Zones. For its breeding haunts it prefers the deep evergreen woods where spruces, firs, and hemlocks predominate, or often swampy woods where the black spruces are thickly draped with Usnea, offering concealment for birds and nests.
In Massachusetts, which is about the southern limit of its breeding range in New England, William Brewster (1888) describes its haunts at Winchendon as follows: “On both high and low ground, wherever there were spruces in any numbers, whether by themselves or mixed with other trees, and also to some extent where the growth was entirely of hemlocks, the Blackburnian Warbler was one of the most abundant and characteristic summer birds, in places even outnumbering the Black-throated Green Warbler, although it shunned strictly the extensive tracts of white pines’ which D. vire’n.s seemed to find quite as congenial as any of the other evergreens.”
Gerald Thayer wrote to Dr. Chapman (1907) that at Monadnock, New Hampshire, it is “a very common summer resident. It is one of the four deep-wood Warblers of this region, the other three being the Black-throated Blue, the Northern Parula and the Canada. While all the other summer Warbiers of Monadnock seem better pleased with various sorts of lighter timber, these four are commonest in the small remaining tracts of primeval woodland, and in the heaviest and oldest second growth. But despite this general community of habit, each of the four has marked minor idiosyncrasies. The Blackburnian favors very big trees, particularly hemlocks, and spends most of its life high above the ground.”
Professor Maurice Brooks (1936) says that Blackburnian warblers “are thoroughly at home in the deciduous second-growth timber that in so many places has replaced the coniferous forest. They range down to elevations of 2,500 feet in northern West Virginia. Here they associate with Golden-winged and Chestnut-sided warblers. A favorite perch is on some chestnut tree that has been killed by the blight.” Rev. J. J. Murray tells me that, in Virginia, it is “common above 1,500 feet, wherever there are conifers.” And Thomas D. Burleigh (1941) says of its status on Mount Mitchell in western North Carolina: “Although not known to breed above an altitude of approximately 5,000 feet, this species is fairly plentiful during the late summer in the fir and spruce woods at the top of the mountain, appearing regularly in July and lingering through September.”
Spring: The Blackburnian warbler is apparently rare in spring in the Atlantic States south of North Carolina; its migration range extends westward to the plains of eastern Texas, eastern Kansas, and eastern Nebraska, but it is rare west of the forested regions of the Mississippi Valley. Professor Cooke (1904) says that the average rate of migration “from the mouth of the Mississippi to its source, where it breeds, appears to be scarcely 25 miles per day.” Forbush (1929) writes:
It Is generally regarded as rare In migration In Massachusetts, though probably untold numbers pass over the state every year, but only a few stop here. It Is not when the birds are migrating that we see them, but when they Btop to rest.
* I can recall but two instances In my lifetime when myriads of Blackburnian Warhiers stopped here, though other similar flights probably have come when I was not there to see. At sunrise one morning in early May, many years ago, when the tiny green leaves were Just breaking forth on the tall trees of the woods near Worcester, Blackburmans were everywhere in the tree-tops. They swarmed In the woods for mIles. Years later, in Amesbury, on another May morning, the night flight, having met a cold wave from the north with a light frost, had come down to earth and the birds were busily looking for food; many Blackburnians and many other warbiers were In the low shrubbery, in the grass, and even on plowed fields in every direction all through the village and about the farms. The sudden cold had stopped them. A few hours later as the day grew warmer they disappeared and were not seen again.
Brewster (1906) says: “We see the beautiful Blackburnian oftenest during the later part of May, in extensive tracts of upland woods, where it spends much of its time in the tops of the larger trees, showing a decided preference for hemlocks and white pines. In Cambridge I have repeatedly observed it in our garden and the immediate neighborhood, usually in tall elms or in blossoming apple trees.”
Nesting: So far as I can learn, the nest of the Blackburnian warbler is almost always placed in a coniferous tree at heights ranging from 5 feet to over 80 feet above the ground; nests have been reported many times in hemlocks, which seems to be a favorite tree, but also in spruces, firs, tamaracks, pines and even a cedar. Ora W. Knight (1908) says: “I have found them breeding in colonies as a rule, that is to say, in a rather dense, mossy carpeted tract of evergreen woods near the pond at Pittsfield [Maine], covering perhaps a square mile, there were about ten pairs of these birds to be found, and in a tract of similar woods about half this size at Bangor there are often six or eight pair nesting. In other words, in suitable localities they tend to congregate in loosely scattered assemblies, while in less suitable spots , genera iiy none, or at most a single pair will be found.” Of a nest found near Winchendon, Mass., Brewster (1888) writes:
The nest, which was found by watching the female, was built at a height of about thirty feet above the ground, on the horizontal branch of a black spruce, some six feet out from the main stem. Its bottom rested securely near the base of a short, stout twig. Above and on every side masses of dark spruce foliage, rendered still denser by a draping of Usnca (which covered the entire tree profusely), hid the nest so perfectly that not a vestige of it could be seen from any direction. This nest is composed outwardly of fine twigs, among which some of the surrounding Usnca is entangled and interwoven. The lining Is of horse hair, fine, dry grasses, and a few of the black rootlets used by D. maculosa. The whole structure is light and airy in appearance, and resembles rather closely the nest of the Chipping Sparrow.
The highest nest of which I can find any record is one reported by Dr. C. Hart Merriam (1885), found by A. J. Dayan in a grove of large white pines (Pinus strobus),in Lewis County, N. Y. It was saddled on a horizontal limb of one of the pines, about 84 feet from the ground and about 10 feet out from the trunk. “The nest is large, substantial, and very compact. It consists almost entirely of a thick and densely woven mat of the soft down of the cattail (Typha latifolia), with seeds attached, and is lined with fine lichens, horse hair, and a piece of white thread. On the outside is an irregular covering of small twigs and rootlets, with here and there a stem of moss or a bit of lichen.”
The lowest nests that I have heard of are recorded in Frederic H. Kennard’s notes from Maine; one was only ~½ feet up and the other 9 feet from the ground in small spruces. Mrs. Nice (1932) found a nest near her mother’s home in Pelham, Mass., that was “18 feet from the ground near the top of a cedar among comparatively open, young growth, 40 yards south of the house and 150 yards to the east of the great pines and hemlocks where the male habitually sang.” The only nest of this warbler that I have ever seen was found by watching the female building it, on June 16, 1913, on an island in Lake Winnipegosis, Manitoba; it was only about 10 feet from the ground, near the end of a drooping branch of a large black spruce that stood on the edge of some coniferous woods next to an open swale. The nest, shaded from above, was partly concealed from below by dense foliage and was, apparently, well made of soft fibers, deeply cul)ped, and lined with some dark material and a little willow cotton. I was not able to visit the island again.
In New York State and in Pennsylvania, the nests of the Blackburnian warbler are almost invariably placed in hemlocks. All of the four nests recorded by T. E. McMullen (MS.) from the Pocono Mountains, Pa., were in hemlocks. And Todd (1940) states that with one exception all the nests found by R. B. Simpson, of Warren, Pa., were in hemlocks, “at elevations varying from twenty to fifty feet. The exceptional nest was in a large chestnut, sixty feet from the ground.”
Dr. Roberts (1936) mentions a Minnesota nest “situated in an arbor vitae tree, directly over the entrance to a cabin,” and one “placed in a small spruce, close to the trunk, about 2 feet from the top of the tree and about 20 feet from the ground. Another was found in “a jack-pine tree, 20 feet from the ground, 6 feet from the trunk, resting in a tangle of small branches, and concealed by a closely overhanging branch.”
Eggs: The Blackburnian warbler lays normally 4 or 5 eggs, usually 4; in a series of 14 sets there are only 3 sets of 5. They are ovate to short ovate and slightly glossy. The ground color is snowy white or very pale greenish white, and is handsomely spotted and blotched with “auburn,” “bay,” “argus brown,” “Mars brown,~~ or “mummy brown,” with undertones of “brownish drab,” or “light vinaceousdrab.” On some eggs the drab marks are the most prevalent, with fewer but more prominent spots or blotches of dark brown shades, such as “Mars brown” and “mummy brown.” Others have spots of “auburn” and “bay” so concentrated that they form a solid band around the large end. In addition a few small scrawls of brownish black are often found. Generally speaking the markings tend to form a wreath, but some eggs are spotted more or less evenly all over the surface. The measurements of 50 eggs average 17.2 by 12.8 millimeters; the eggs showing the four extremes measure 18.0 by 13.6, 17.0 by 13.7, 15.6 by 12.5, and 17.1 by 12.0 millimeters (Harris).
Young: We have no information on incubation and very little on the care of the young. The male has been seen to go onto the nest, and evidently shares occasionally in the duty of incubation. Both parents help in feeding the young, as noted by Mrs. Nice (1932) at the nest she was watching. When Mrs. Nice’s daughter climbed a tree near the nest, the female “assumed a peculiar attitude, her tail outspread and dropped at right angles to her body, her wings flipping rapidly and occasionally held stiffly up or down. The excitement caused the young to jump out on the ground where they could not be found.”
Plumages: Dr. Dwight (1900) calls the natal down sepia-brown, and in speaking of the males, describes the juvenal plumage as “above, dark sepia-brown obscurely streaked on the back with clove-brown. Wings and tail clove-brown edged with olive-buff, the tertiaries and coverts with white forming two wing bands at tips of greater and median coverts; the outer three rectrices largely white. Below, white, washed with wood brown or buff on breast and sides, spotted, except on chin, abdomen and crissum, with dull sepia. Superciliary stripe cream-buff, spot on upper and under eyelid white; lores and auriculars dusky.”
A partial postjuvenal molt begins early in August, involving the contour plumage and the wing coverts but not the rest of the wings or the tail. This produces the first winter plumage, which he describes as “above, deep yellowish olive-gray, flecked on the crown and streaked on the back with black; obscure median crown stripe straw-yellow; rump and upper tail coverts black, edged with olivegray. Wing coverts clove-brown edged with olive-gray and tipped with white forming two broad wing bands. Below, straw-yellow brightening to orange-tinged lemon on the throat, fading to huffy white on the crissum and narrowly streaked on the sides with black veiled by yellow edgings. Superciliary stripe and postauricular region lemon-yellow orange-tinged. Auriculars, rictal streak and transocular stripe olive-gray mixed with black. Suborbital spot yellowish white.”
He says that the first nuptial plumage is “acquired by a partial prenuptial moult which involves mdst of the body plumage (except posteriorly), the wing coverts and sometimes the tertiaries but not the rest of the wings nor the tail. The full orange and black plumage is assumed, young and old becoming practically indistinguishable, the orange throat equally intense in both, the wings and tail usually browner in the young bird and the primary coverts a key to age.”
The adult winter plumage is acquired by a complete postnuptial molt in July, and “differs little from the first winter dress, but the yellow more distinctly orange, the transocular and rictal streaks, the crown and auriculars distinctly black, veiled with orange tips, the streaking below heavier and broader, the wings and tail blacker and the edgings grayer.” The adult nuptial plumage is acquired as in the young bird; this molt evidently begins in February, while the birds are in their winter quarters, and is usually finished before they reach their summer homes.
Of the females, Dr. Dwight says:
The plumages and moults correspond to those of the male. In juvenal plumage the wing edgings are usually duller, the first Winter plumage being similar to that of the male but browner, the yellow tints nearly lost and the streakings obscure and grayish. The first nuptial plumage, assumed by a more or less limited preauptial moult, is grayer above and paler below, except on the chin and throat where new pale orange feathers contrast with the worn and faded ones of the breast. The adult winter l)lumage is practically the same as the male first winter, the auriculars and transocular stripe usually duller. The adult nuptial plumage is brighter below than the first nuptial and with more spotting on the crown, but the black head and bright orange throat of the male are never acquired.
Food: The Blackhurnian warbler is mainly insectivorous like other wood warblers, feeding almost entirely on the forest pests that are so injurious to the trees. F. H. King (1883), writing of its food in Wisconsin, says: “Of nine specimens examined, four had eaten nine small beetles; five, nineteen caterpillars; one, ants; and one, small winged insect. In the stomachs of three examined collectively, were found four caterpillars, four ants, one dipterous insect .09 of an inch long, one medium sized heteropterous insect, four large crane-flies, and one ichneumon-fly (?). Another bird had in its stomach one heteropterous insect (Tin gis), nine small caterpillars, two leaf-beetles, and two large crane-flies.”
Ora W. Knight (1908) writes: “In general I have found large quantities of the wing cases and harder body portions of beetles in the stomachs of such Blackburnian Warblers as I have dissected, also unidentifiable grubs, worms, larvae of various lepidopterous insects and similar material. As a rule they feed by passing from limb to limb and examining the foliage and limbs of trees, more seldom catching anything in the air.
R. W. Sheppard (1939), of Niagara Falls, Ontario, observed a male Blackburnian warbler in his garden for several days, November 5 to 11, 1938, that appeared to be traveling with two chickadees, among some willow trees. “An examination of the row of low willow trees which appeared to be so attractive to this particular warbler, revealed the presence of numbers of active aphids and innumerable newly laid aphis eggs, and it is probable that these insects and their eggs provided the major incentive for the repeated and prolonged visits of this very late migrant.”
Henry D. Minot (1877) observed “a pair feeding upon ivy berries” on April 21, when insects were not yet common in Massachusetts.
Behavior: William Brewster (1938) describes what he thought was the unique behavior of a female Blackburnian warbler at its nest, although a similar habit has been observed in other wood warblers. Even though the eggs “were perfectly fresh the female sat so closely that thumping and shaking the tree (a slender one) failed to start her, and when Watrous climbed it he nearly touched her before she slipped off. She then dropped like a stone to the ground over which she crawled and tumbled and fluttered with widespread tail and quivering wings much like a Water Thrush or Oven Bird and evidently with the hope of leading us away from the nest.”
The Blackburnian is preeminently a forest warbler and a treetop bird. On migrations it frequents the tops of the trees in the deciduous forests, often in company with other wood warblers; and on its breeding grounds in the coniferous forests the male loves to perch on the topmost tip of some tall spruce and sing for long periods, his fiery breast gleaming in the sunlight. As his mate is probably sitting on her nest not far away, his serenity may be disturbed by the appearance of a rival; but the intruder in his territory is promptly driven away and he resumes his singing.
Voice: Aretas A. Saunders has sent me the following study: “The song of the Blackburnian warbler is one that is usually of two distinct parts, the first a series of notes or 2-note phrases all on one pitch, and the second a faster series, or a trill, on a different pitch. It is very high in pitch, with a thin, wiry quality, rather unmusical, and not loud but penetrating.
“Of my 34 records, 25 have the second part higher in pitch than the first, while in the other 9 it is lower. I do not think, however, that this means that the higher ending is commoner, for there is reason to think that the difference is geographical. Of 11 records of migrating birds in Connecticut, 10 end in the higher pitch. Of 15 records from breeding birds in the Adirondacks, 13 end in the higher pitch; but of 8 records of breeding birds in Allegany State Park in western New York, only 2 end in the higher pitch, and 6 in the lower.
“In 20 of the records the first part. of the song is of 2-note phrases, but the remainder is of single repeated notes. In 6 records, ending in a higher pitch, the final trill slurs upward in pitch, suggesting the ending of a typical parula song in form. In 10 of the records the second part is much shorter than the first.
“Songs vary from 1% to 22/s secoiids, averaging a little longer than those of other species of this genus. The number of notes in songs, excepting those with trills, varies from 7 to 25, and averages 14. Pitch varies from D”” to F””, one and a half tones more than an octave. It ranks with the blackpolled and bay-breasted warblers in the very high pitch of its upper notes but shows more variation in pitch than either.
“The song of this bird ceases earlier in summer than most others. In 14 summers in Allegany Park, the average date of the last song was July 12, the earliest July 4, 1029, and the latest July 22, 1935. I have never heard singing in late summer after the ~nolt.”
Francis II. Allen sends me his impressions as follows: “Like so many of our warblers, the Blackburnian has two song-forms, but both are subject to great individual variation. An extremely high note is almost an invariable characteristic. In one form it is the closing note, and in the other it ends each repeated phrase of a succession that constitutes the main part of the song. The first song resembles that of the parula, but ends with this high note, while the main part is less buzzy and more what I might call pebbly in character. The second I have been accustomed to call the chickawee song because of the repeated phrase which suggests those syllables. At Sherburne, Vt., in June, 1907, I found the Blackburnians singing a song that I rendered as chi-ee chi-ee aid-es aid-es chip. Another rendering of the same or a similar song, recorded at Jaifrey, N. H., May 30, 1910, was eerw~e serw& serwke serwAc .serwip, with the emphasis on the wip. At New London, N. H., in June, 1931, where this was perhaps the commonest of the warbiers, I was particularly impressed by the variability of both the songs. In some, the very high and attenuated notes were so short that for some time I failed to recognize their source. One bird sang Middle chiddle chiddle chick-a chick-a cheet. At Hog Island in Muscongus Bay, Maine, in June, 1936, I heard a song of which only a sweet weet weet weet weet carried to a distance, but of which, heard near at hand, the end was found to be a short, confused succession of high-pitched, dry notes concluding with a very high, short note. This was, I think, the most pleasing performance I have ever heard from this species.
Mrs. Nice (1932) mentions three different songs; the commonest and shortest, like the parula’s in form, lasts for one second and is given at intervals of ~½ to 10 seconds; the rarest and longest lasts for two seconds and is given at intervals of 10 or 14 seconds.
A. D. DuBois tells me that the Blackburnian warbler “has a song not unlike that of the dickeissel in its general form, although much subdued in volume.” Gerald Thayer wrote to Dr. Chapman (1907) of two or more different songs of this warbler, and says:
Its voice is thin, but, unlike the Parula’s, exquisitely smooth, in all the many variations of its two (or more) main songs. * * Even the tone quality is not quite constant, for though it never, in my experience, varies toward huskiness, it does occasionally range toward full-voiced richness. Thus I have beard a Blackburnian that began his otherwise normal song with two or three clear notes much like those of the most full and smooth-voiced performance of the American Redstart’s, and another that began so much like a Nashville that I had to hear him several times, near by, to he convinced that there was not a Nashville chiming in. Sometimes, again, tone and delivery are varied toward excessive languidness; and sometimes, contrariwise, toward sharp, wiry
Enemies: Dr. Friedmann (1929) calls the Blackburnian warbler “a very uncommon victim of the Cowbird.” Dr. Merriam (1885) records a nest of this warbler that was 84 feet from the ground, containing four warbler’s eggs and one of the cowbird, of which Friedmann remarks: “This is probably the a]titude record for a Cowbird’s egg, bettering by some twenty feet my highest record at Ithaca, a Cowbird’s egg in a nest of a Pine Warbler about sixty feet up.”
Harold S. Peters (1936) records two species of lice, Menacanthus chrysophacum (Kellogg) and Ricinus pallens (Kellogg), and one mite, Proctophyllodes sp., as external parasites of this warbler.
Field marks: The adult male Blackburnian warbler in spring plumage is unmistakable, with its black upper parts, large white patch in the wings, orange stripe in center of the crown and another over the eye, and, especially, the flaming orange throat and breast. The female in the spring and the male in the fall are similarly marked, but the colors are much duller. The colors of young birds in the fall are even duller, and the back is brownish, but the white outer web of the basal half of the outer tail feather should indicate the species.
Fall: Early in August, young and old birds begin to gather into flocks preparing to migrate, and before the end of that month most of them have left their breeding grounds. All through August and most of September, we may see them drifting through our deciduous woods in mixed flocks with other species of warblers. These migrating flocks are generally so high up in the tree tops and are so active in their movements that it is not easy to identify them in their dull winter plumages.
By early October, most of the Blackburnian warblers have passed beyond the United States, en route to their winter home in South America. Professor Cooke (1904) says: “By the middle of October the earliest migrants have reached Venezuela and Ecuador. The main army of the Blackburnians pass the south end of the Alleghenies between September 25 and October 5, and during the first two weeks of October are moving through San Jos~, Costa Rica, and by early in November are settled for the winter in Per6.”
Dickey and van Rossem (1838) refer to it as a “fairly common fall migrant and very rare winter visitant in the Arid Lower Tropical Zone” in El Salvador, but “not seen in spring.~~ Winter: Dr. Alexander F. Skutch contributes the following notes: “Rarely recorded, and apparently only as a bird of passage, in Guatemala, the Blackburnian warbler is a moderately abundant winter resident in Costa Rica. Here it passes the winter months on both slopes of the Cordillera, from about 1,500 to 0,000 feet above sea-level, but is far more abundant above than below 3,000 feet. It is found in mid-winter both in heavy forest and among scattered tall trees. Although the birds appear to arrive in flocks in late August or September, they soon disperse through the woodland and show slight sociability. Yet one or two may at times join a mixed flock of Tennessee warblers and other small birds. Restlessly active, the Blackburnian warbler forages well above the ground, ‘where it is difficult to see. I have never heard its song in Central America.
“Early dates of fall arrival are: Guatemala: Chimoxan (Griscom), October 1; Panajachel (Griscom), October 4. Costa Rica: San Jos6 (Cherrie), September 8; San Jos6 (Underwood), Septem 10; La Hondura (Carriker), September 19; San Isidro de Coronado, September 8, 1935; Vara Blanca, August 19, 1937; Cartago, September 13, 1938; Murcia, September 14, 1941; Basin of El General, September 16, 1936; Ujarr~s (Carriker), September 12. Ecuador: Volc~n Tungurahua, 7,400 feet, October 12, 1939.
“Late dates of spring departure from Central America are: Costa Rica: Basin of El Genera], March 25, 1936, March 13, 1937 and April 18, 1943; Vara Blanca, May 7, 1938; Pejivalle, April 23, 1941; Bonilla (Basulto), April 10. Guatemala: Finca Sepacuite (Griscom), May 10.” DISTRIBUTION
Range: Southern Canada east of the Great Plains to Central Peru. Breeding range: The Blackburnian warbler breeds north to southern Manitoba (Lake St. Martin and Berens Island, Lake Winnipeg); central Ontario (Lac Seul, Lake Abitibi, and North Bay; has occurred at Trout Lake); and central Quebec (Blue Sea Lake, Lake Albanel, rarely; Lake St. John and Gasp6; possibly Pointe de Monts and Natasliquan). East to eastern Quebec (Gasp6); eastern New l3runswick (Bathurst and Tabusintac) ; and eastern Nova Scotia (Antigonish and Halifax). South to Nova Scotia (Halifax) ; southern Maine (Calais, Lewiston, and Portland); Massachusetts (Cambridge, Springfield, and Sheffield); northern New Jersey (Kittatinny Mountains) ; central Pennsylvania (Mauch Chunk and Carlisle); and south through the mountains of Maryland, Virginia, West Virginia, North and South Carolina and Tennessee to northern Georgia (Brasatown Bald and Burnt Mountain); western Pennsylvania (Leasureville and Meadville); northeastern Ohio (Pymatuning Swamp and possibly Geneva); northern Michigan (Bay City and Wequetonsing); northern Wisconsin (New London, Unity and Ladysmith); and northern Minnesota (Elk River, Onamia, and Itasca Park). West to northwestern Minnesota (Itasca Park) and southeastern Manitoba (Winnipeg and Lake St. Martin).
A possible future extension of range westward is seen in records from Saskatchewan: it was recorded four times near Indian Head 1888: 1001; one at Last Mountain Lake in 1920, at Lake Johnston in 1922; and at Emma Lake in the summer of 1939, possibly breeding.
Winter range: The Blackburnian warbler is reported to winter commonly in Costa Rica, but as yet has been found in Panama only as a migrant. In South America it is found north to northern Colombia (Santa Marta region) ; and central northcrn Venezuela (Rancho Grande). East to northwestern Venezuela (Rancho Grande, M~rida, and P6.xamo de Tam~i); the eastern slope of the Andes in Colombia (Pamplona, Bogota, and San Antonio); Ecuador (Mount Sumaca, Machay, and Zamora); and Peru (Chinchao and Huambo). South to central Peru (Huambo and Anquimarca). West to western Peru (Anquimarca and Tambillo); Ecuador (Ambato, Quito, and Parambo); and Colombia (Concordia, Medellin, and the Santa Marta region). It is casual in migration in the Bahamas and Cuba.
Migration: Late dates of spring departure from the winter home are: Perni: Chelpes, April 22. Ecuador: Quito, May 10. Vanezuela: Rancho Grande, April 22. Colombia: La Porquera, April 24. Costa Rica: Vera Blanca, May 7.
Early dates of spring arrival are: Panama: Garachin~, March 5. Florida: Pensacola, April 5. Alabama: Hollins, April 4. Georgia: . Athens, March 29. South Carolina: Aiken, April 17. North Carolina: Weaverville, April 16. Virginia: Lynchburg, April 25. West Virginia: White Sulphur Springs, April 17. District of Columbia: Washington, April 23. Pennsylvania: Renovo, April 27. New York: Rochester, April 26. Massachusetts: Melrose, April 29. Vermont: Wells River, April 30. Maine: Portland, May 4. New Brunswick: Scotch Lake, May 5. Quebec: Montreal, May 10. Louisiana: Lake Borgne, March 27. Mississippi: Gulfport, March 27. Tennessee: Chattanooga, March 31. Kentucky: Lexington, April 12. Indiana: Brookville, April 15. Ohio: Oberlin, April 19. Michigan: Hillsdale, April 22. Ontario: London, April 27. Arkansas: Huttig, April 15. Missouri: Bolivar, April 20. Iowa: Davenport, April 28. Wisconsin: Unity, April 27. Minnesota: Waseca, April 30. Texas: Boerne, March 31. Nebraska: Stapleton, May 1. South DakotaVermilion, May 3. Monitoba: Aweme, May 14.
Late dates of spring departure of transients are: Florida: Pensacola, May 9. Alabama: Autaugaville, May 12. Georgia: Athens, May 7. South Carolina: Spartanburg, May 12. North Carolina: Greensboro, May 17. Virginia: Charlottesville, May 28. District of Columbia: Washington, June 3. Pennsylvania: Norristown, May 30. New York: New York, June 7. Louisiana: New Orleans, April 23. Mississippi: Corinth, May 12. Kentucky: Lexington, May 16. Illinois: Lake Forest, June 9. Ohio-Toledo, June 12. Arkansas: Rogers, May 12. Missouri: Kansas City, May 30. lowa: Sigourney, June 1. Texas: Commerce, May 18. Nebraska: Fairbury, May 26. South Dakota: Yankton, June 2.
Late dates of fall departure are: Saskatchewan: Last Mountain Lake, September 1. Nebraska: Fairbury, October 14. Texas: Brownsville, October 2. Minnesota: Saint Paul, September 25. Wisconsin: Madison, September 27. Ontario: Hamilton, October 3. Michigan: Ann Arbor, October 8. Indiana: Waterloo, October 17. Kentucky: Danville, October 16. Missouri: St. Louis, October 6. Tennessee: Memphis, October 28. Arkansas: Chicat, October 4. Mississippi: Eudora, October 24. Louisiana: New Orleans, October 9. Quebec: Hatley, September 30. New Brunswick: Scotch Lake, September 28. Maine: Phillips, September 17. New Hampshire: Hanover, September 24. Massachusetts: Welles]ey, October 23. New York: Canandaigua, October 12. Peunsylvania: Berwyn, October 19. District of Columbia: Washington, October 10. West Virginia: Bluefleld, October 8. Virginia: Sweet Briar, November 1. Georgia: Tifton,November 2. Alabama: Birmingham, October 25. Florida: Arcadia, October 30. Cuba: Bosque de Ia Ilabana, October 30.
Early dates of fall arrival are: North Dakota: Argusville, August 23. Texas: Commerce, August 28. Illinois: Glen Ellyn, August 19. Ohio: Little Cedar Point, July 31. Kentucky: Versailles, August 31. Tennessee: Nashville, August 29. Mississippi: Bay St. Louis, August 11. New York: New York, August 11. Pennsylvania: Berwyn, August 19. District of Columbia: Washington, August 2. Virginia: Charlottesville, August 10. North Carolina: Mount Mitchell, July 30. Georgia: Savannah, August 10. Florida: Key West, July 29. Cuba: Santiago de las Vegas, September 20. Costa Rica: San Jos6, August 17. Colombia: Santa Isabel, September 22. Venezuela: Escorial, October 14. Ecuador: Tumbaco, October 12. Perfi: Tambillo, November 19.
Casual records: A specimen of Blackburnian warbler was collected at Frederickshaab, Greenland, on October 16, 1845. One was taken at Ogden, Utah, in September 1871, and another near Fort Bayard, N. M., in May 1876. On August 21, 1924, a male was watched closely for sometime near Libby, Mont.
Egg dates: Maine: 5 records, June 2 to 17.
New York: 23 records, May 29 to July 6; 16 records, June 7 to 17, indicating the height of the season.
New Hampshire: 6 records, May 23 to June 18.
Pennsylvania: 5 records, May 28 to June 9. Quebec: 2 records, June 15 and 20.


